Describe a Liquid in Terms of Shape and Volume
Assumes the shape of the part of the container which it occupies particles can moveslide past one another. Motion of 1 particle is unaffected by motion of other particles unless they collide.

The Particulate Nature Of Matter Igcse Cambridge 2019 2021 Teaching Resources States Of Matter Matter Science Matter Worksheets
Color shape odor physical state melting point boiling.

. The intermolecular forces are practically non-existent. Some Characteristics of Gases Liquids and Solids and the Microscopic Explanation for the Behavior. A solid has a constant volume and a determinate shapeA liquid has a constant volume but an indeterminate shape.
Are solid liquid and gas. Solids have a fixed shape and occupy a fixed volume. Fixed shape and volume.
Indefinite shape and volume. Properties observable without changing composition. A gas lacks either a defined shape or volume.
A It has a definite shape and a definite volume. Liquids because they flow can occupy whatever shape their container has so they do not have a fixed shape. Because the particles in liquids are very close together barely further apart than in solids liquids do not easily compress so their volume is fixed.
The four main states of matter are solids liquids gases and plasma. Liquids shape volume c. Liquids are difficult to compress as particles have less space between them to.
Under exceptional conditions other states of matter also exist. A liquid has a definite volume but takes the shape of its container. Matter in the liquid form has a defined volume but no defined shape.
The particle model represents particles by small solid spheres. Which of the following describes the liquid phase. Matter has mass and takes up space.
Assumes the shape and volume of its container particles can move past one another. Liquid is a substance that flows freely having a definite volume but no permanent shape. Particles are free to move over each other but are still attracted to each other.
Thus there is no definite volume. Describe solids liquids and gases in terms of their shape and volume as definite or indefinite a. D It has neither a definite shape nor.
The following are the characteristics of the liquid-state matter. Liquids have a predetermined or fixed volume. Liquids take the shape of the container in which they are kept.
There are four states of matter solid liquid gas and plasma. It is the only metal we know of that is liquid at room temperature. A solid has a definite shape and volume.
B It has a definite shape but not a definite volume. Gases do not have a definite shape. Retains a fixed volume and shape.
Gas refers to a state of matter do not have any shape but conform to the shape of the container completely in which it is put in. Liquids do not have a defined form. The intermolecular space between solids is absent.
Particles in a gas are in constant random motion. The three states of matter. Process in which a substance changes its physical appearance but not its chemical composition.
A gas has an indeterminate volume. A solid has a constant volume and a determinate shapeA liquid has a constant volume but an indeterminate shapeA gas has an indeterminate. Gases shape volume 2.
Solids shape volume b. Neither definite shape nor volume. Describe the term physical property and chemical property.
No fixed shape but has volume. They may be crushed and are not. No definite shape takes the shape of its container has definite volume.
It describes the arrangement movement and. Whenever the matter has a melting point above room. A familiar liquid is mercury metal.
They may be crushed and are not stiff. Gases can also flow so occupy the shape of their whole. C It has a definite volume but not a definite shape.
They take on the shape of the container theyre stored in. Forces of attraction among particles in a gas can be ignored under ordinary conditions. Liquids consist of atoms or molecules that are connected by intermolecular bonds.
Solids have a definite shape to them. Provide two 2 examples of physical properties and two 2. A liquid is one of the states of matter.
Liquids do not have a definite shape. In a liquid state of matter particles are less tightly packed as compared to solids. Mercury is an anomaly.
How may liquids be described in terms of shape and volume. All the constant motions of particles in a gas allows the gas to fill a container of any shapesize. Liquids have the following characteristics.
Assumes shape volume of its container. Definition of a Liquid in terms of Shape Volume and Compressibility. The particles in a liquid are free to flow so while a liquid has a definite volume it does not have a definite shape.

David Chalk Teacherchalky1 Twitter Gcse Science Revision Gcse Science Science Revision

Solid Liquid Gas Phases Changes Venn Diagram Sorting Activity Public 2 Venn Diagram Activities Venn Diagram Matter Science
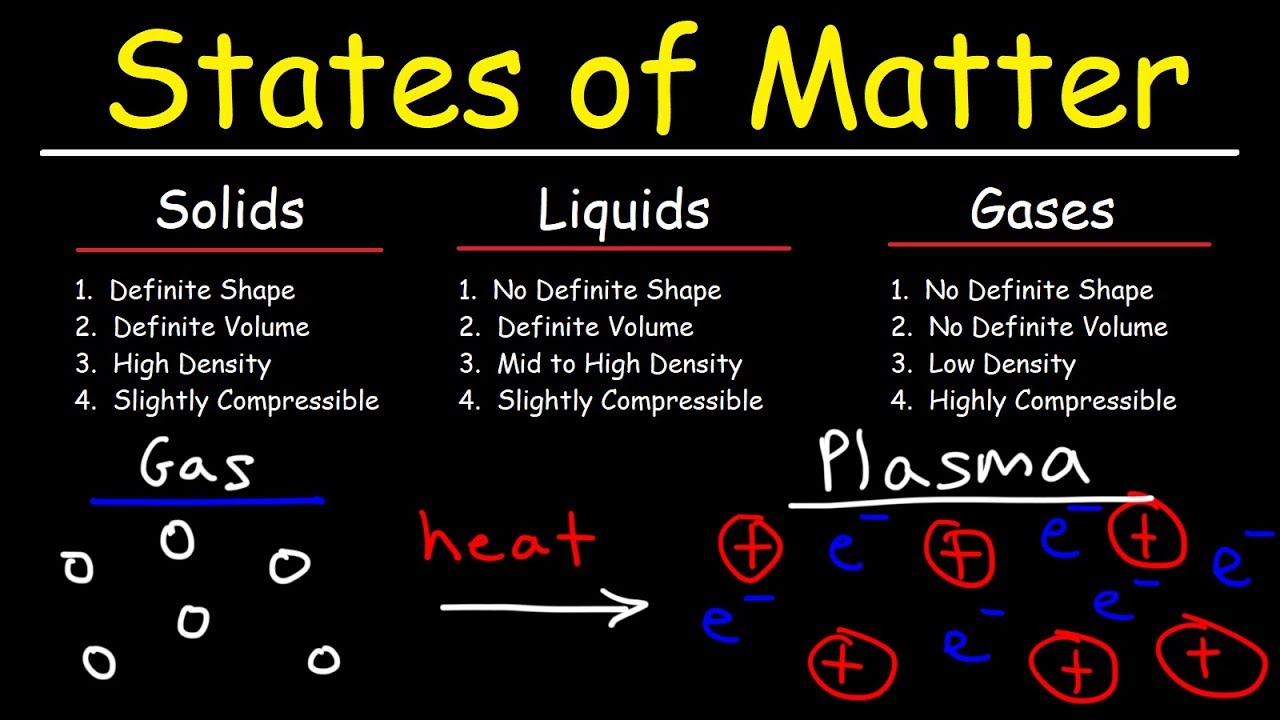
States Of Matter Solids Liquids Gases Plasma Chemistry Youtube
Comments
Post a Comment